Day 2 :

Biography:
Andreas Weiler is the Global Business Unit Head of Emerging Technologies at Lonza where he oversees the Cell and Gene Therapy Contract Manufacturing businesses. Before joining Lonza he worked for Sigma Aldrich (SAFC), another leading CDMO (Contract Development and Manufacturing Organization) to the Pharma/biotech industries, for over 17 years where he held several positions as Business Director and finally as the Head of Strategic Marketing to the organization. Since 2015, when Andreas joined Lonza, Cell and Gene Therapy sales have more than doubled and the manufacturing footprint expanded significantly to cover the globe with facilities in North America, Europe and Asia. Andreas holds a PhD in Organic Chemistry from University of Freiburg and an MBA degree.
Abstract:
Cell and Gene Therapies are seen as the next frontier in medicine as they have the potential to bring cures to patients that suffer from life-threatening diseases without many treatment options available to them. The idea that a patient’s own cells can be reprogrammed to replace or eliminate faulty genes or to attack cancer cells in a way that is not naturally possible is giving hope to many. In 2017, we have seen several approvals from these innovative medicines, Kymriah, Yescarta and Luxturna to name a few. However, a key challenge for all players and drug-makers in this field remains to be addressed: the cost of manufacturing is critically high due to the nature of these highly personalized medicines. This high cost greatly limits the number of patients that can be eligible and threatens the sustainability of the therapy as a whole, and this is likely to be reflected in the already high price tags of these drugs for the foreseeable future. In his presentation, Andreas Weiler would like to show the way these medicines are manufactured and delivered to the patients could have significant and disruptive implications for the future of healthcare. He will be sharing his vision and innovative technologies that could reduce the cost of these medicines significantly and make them accessible to a larger number of patients.

Biography:
Jacques Beckmann is the Head of clinical bioinformatics at the Swiss Institute of Bioinformatics (SIB) from December 2012 till December 2016. He served previously for ten years as Professor and Chairman of the Department of Medical Genetics at the Faculty of Biology and Medicine of the Univ. of Lausanne as well as head of the Medical Genetics Service of the Centre Hospitalier Universitaire Vaudois (CHUV). Previously, he held a chair as Professor at the Dept of Molecular Genetics at the Weizmann Inst of Science, Rehovot, Israel. Initially trained in molecular genetics he then moved to genetics. In the 1980s, together with Prof. M. Soller from the Hebrew Univ, they pioneered the use of marker-assisted genetic improvement in plants and animals, focusing on Quantitative Trait Loci (QTLs). His interest shifted in 1990 to human genetics with a move to Paris, where he held successively senior research positions at the CEPH, Généthon (Evry), and finally the Centre National de Génotypage (CNG, Evry), where he was Deputy-Director. During those years he collaborated with Prof. D. Cohen, J. Weissenbach, M. Lathrop, J. Dausset and others and contributed significantly to the elaboration of genetic, physical and gene maps of the human genome, as well as to the positional cloning of a number of disease loci, many of which are involved in muscular dystrophy. Prof. Beckmann has published over 390 scientific peer-reviewed articles in molecular genetics, genetics and genomics, and has an ISI H-index of 86. He has served on the editorial boards of a number of scientific journals. His research interests include genomic disorders, pharmacogenetics as well as the genetic basis of complex traits.
Abstract:
The recent years have seen the emergence of both ground-breaking scientific developments in high-resolution, high-throughput data gathering technologies enabling cost-effective collection and analysis of huge, disparate datasets on individual health, as well as of sophisticated clinical bioinformatics or machine learning tools required for the analyses and interpretation of this wealth of data. These developments have triggered numerous initiatives in precision medicine (PM), a data-driven and currently still, essentially a highly genome-centric initiative. Proper and effective delivery of PM poses numerous challenges. Foremost, PM needs to be contrasted with the powerful and widely used practice of evidence-based medicine (EBM). The latter is informed by meta-analyses or group-centered studies from which mean recommendations are derived. These amount at first approximation to a “one size fits all” approach, whose major limit is that it does not provide adequate solutions for outliers. Yet, we are all outliers for one or another trait. In contrast to EBM, one of the strengths of PM, which focuses on the individual, lies in the area of individualized management, and this includes outliers.To achieve these objectives, it will be necessary to bridge PM and EBM. Through the collection, analyses and sharing of standardized medically relevant data globally, evidence-based PM will shift progressively from therapy to prevention, thus leading eventually to improved, clinician-to-patient communication, citizen-centered healthcare and sustained well-being. We will discuss challenges and opportunities towards these goals.
Stephen Harlin
The Harlin Center for Genomic Medicine, USA
Title: Implementing Genomics in a Precision Medicine Practice

Biography:
Abstract:
A tremendous opportunity exists for implementing genomics into clinical practice. However, there is little research on genomics-based practice protocols or clinical decision support systems (CDSS’s) that incorporate genomic information technologies at the point-of-care.
We developed a CDSS whose user interface combines raw DNA sequence variant data and gut microbial biomarkers to enable a high level, holistic interpretation of the holobiome. Our goal was to individualize therapeutic and preventive interventions, based on insights gleaned from two ‘omics’ data sources and advanced laboratory biomarkers for oxidative stress and inflammation.
While several studies have shown that polygenic risk analysis accurately predicts the individual risk of developing a chronic disease, our model attempts to extend risk stratification to identify molecularly defined subsets of individuals. Specifically, our CDSS clusters polymorphisms in multiple genetic loci that contribute to the pathogenesis of many diseases: glucose and lipid dysregulation, endothelial dysfunction, mitochondrial stress, deficiencies in DNA repair capacity, circadian disruption, maladaptive emotion regulation, and a dysbiotic gut microbiome.
We believe that intelligent decision support tools are crucial to the integration of heterogenous inputs from ‘omic’ technologies and evidence-based clinical regimens. Towards that end, we have designed an application that allows for easy, reliable, and rapid assessment of genotype-phenotype relationships. Output is relevant and intelligible to both physicians and patients. In short, our vision of a genomic CDSS fully embraces the concept of the clinical holobiome.
Finally, we present a descriptive case study of a 58-year-old woman with a history of thrice recurrent bladder cancer. The presentation demonstrates how genetic loci, likely representing disease mechanistic pathways, can be paired with microbiota datasets and interpreted in the context of other known risk factors. This comprehensive approach provides new prospects for disease management and prevention.
Rizwan Ya. Abdullaiev
Kharkiv National Medical University, Ukraine
Title: Transvaginal ultrasound characteristics of the uterus and ovaries in young women with polycystic ovaries

Biography:
Rizvan Abdullaev is currently working in the Kharkiv National Medical University Ukraine. He has published numerous research papers and articles in reputed journals and has various other achievements in the related studies. He has extended his valuable service towards the scientific community with his extensive research work.
Abstract:
The aim of the study was the echographic semiotics of PCOS in young women in a transvaginal triplex mode.
Materials and Methods: The study included the results of transvaginal ultrasound examination 193 female with menstrual irregularities, aged 18–27 years were recruited in the study. Transvaginal echography (TVE) was performed on the 4th-6th and 8th-10th days of the menstrual cycle, and in the absence of menstruation on any day. All women had the ultrasound criteria of PCO: the presence of 12 or more 2-9 mm ovarian follicles and ovarian volume of more than 10cm3. Considering the distribution of follicles in the ovaries and their volume, the blood flow in the stroma, the level of antimulylerovogo hormone, the ratio luteinizing and follicle-stimulating hormones (LH / FSH), polycystic ovary syndrome (PCOS) was diagnosed in 139 (72.0%) women, multifollicular ovaries (MFO) in 54 (28.0%) women.
Results: Peripheral location of ovaries in women with PCOS was noted in 78 (56.1 ± 4.2%) cases, mixed in 61 (43.9 ± 4.2%) cases (P <0.05), in patients with MFO in 23 (42,6 ± 6,7)%) and in 31 (57,4 ± 6,7%) cases, and among the fertility healthy women in 27 (79,4 ± 6,9%) and in 7 (20,6 ± 6.9%) cases respectively (P <0.001). An enhanced in stromal echogenicity was noted in 74 (53.2 ± 4.2%) of women with PCOS, in 17 (31.5 ± 6.3%) women with MFO (P <0.01).
The number of follicles within the limits of 13-15 was registered in 64 (46.0±4.2%) of women with PCOS, 38 (70.4±6.2%) of women with MFO (P<0.001), and more than 15 follicles - in 75 (54.0±4.2%) and in 15 (27.8±6.1%) women (P<0.001), respectively.
In the group of PCOS, the average volume of the ovaries was 18.1±3.8cm3, in the group of MFO – 12.3±2.8cm3, respectively. There were no statistically significant differences in the volumes between these groups. However, in the both groups the volume of the ovaries was significantly (P <0.01) higher than the parameters of the control group.
The vascularization of the ovary stroma in the patients with PCOS was significantly more than in the MFO. The average value of Vmax in the group of PCOS was 49.1±8.6cm/s, in the group of MFO - 35.6±7.1cm/s, in fertility healthy women - 18.9±4.6cm/s, respectively (P<0.001 and P<0.05). Parameters of resistances index (RI) in these groups were 0.48±0.03; 0.54±0.03 and 0.54±0.03, respectively (P<0.05).
The average length of the cervix in the group of women with PCOS was 43.7 ± 3.6 mm, in the group with MFJA - 34.1 ± 3.2 mm, in healthy women - 28.6 ± 2.9 mm, respectively.
The average uterine body length in the group of women with PCOS was 43.7 ± 3.6 mm, in the group with MFJA - 34.1 ± 3.2 mm, in healthy women - 28.6 ± 2.9 mm, respectively.
The ratio of the length of the cervix and the body of the uterus was 1.12 ± 0.07; 0.92 ± 0.06 and - 0.69 ± 0.05, respectively.
Conclusion:
1. Mixed type distribution of follicles, their number is more than 15 on the echographic section, the volume of the ovary is more than 14 cm3, an increase in the number of color vascular signals, an increase in the maximum systolic velocity of more than 50 cm/s, a decrease in peripheral resistance to blood flow of less than 0.51 increases the reliability of ultrasound PCOS criteria.
2. In the syndrome of polycystic ovaries, uterine hypoplasia is observed, which is manifested by an increase in the length of the cervix and an increase in the ratio of the length of the cervix and the body of the uterus.
Getu Molla Tarekegn
Nutrition at Micronutrient Initiative,Ethiopia
Title: Integrating micronutrient interventions into health systems for program sustainability; a case study on vitamin A supplementation (VAS) in Ethiopia.

Biography:
Getu Tarekegn has expertise on research, emergency preparedness & response plan, project design, implementation, monitoring and evaluation, and proposal writing. In line with MI’s strategic objectives in Africa, he contributed to improved child health and nutrition in Ethiopia by supporting the Federal Ministry of Health in the implementation of effective, integrated and sustainable vitamin A supplementation. He has contributed a lot in child survival interventions, community based nutrition program and in the development of nutrition related policies and strategies in Ethiopia. Getu has been coordinating the integration of vitamin A supplementation program from more vertical program into the routine health system in Ethiopia. Considerable advances have been made in integrating VAS along wide range of health system dimensions including policy, financing, planning, training, supply management, supportive supervision and M&E. Children aged 6-59 months that received two doses of vitamin A through integrated VAS delivery strategies has been maintained at above 80%.
Abstract:
Statement of the Problem: Integration of micronutrient interventions into a country’s health system is a means for achieving sustainability of high impact and cost-effective interventions. The biggest challenge with integration is to maintain high coverage achieved through less integrated interventions. To address this concern, Micronutrient Initiative (MI) developed a framework for integrating micronutrient interventions into health systems. The framework was used in the design of a 4-year program (2011-2015) on integrating vitamin A supplementation into the Ethiopian health system to maintain the high VAS coverage which had been achieved through non integrated intervention. The purpose of this paper is to examine the extent to which VAS has been integrated into the Ethiopia health system, & trends in VAS coverage achieved through integrated delivery, and the lessons learnt. Methodology: A situation assessment on the extent of integration of VAS into the Ethiopian health system was done using the MI’s framework for integration to determine the baseline and set targets. The framework was adopted from WHO Health System Building Blocks which includes; service delivery, human resource, procurement and supplies, M&E, financing, Stewardship & Governance. Findings: Strong health system support at all levels allowed relatively high VAS coverage to be maintained while shifting to an integrated routine system. The integration score has improved from 17 to 25 point score during the four years of program implementation. Conclusion & Significance: The MI’s framework for integrating micronutrients into health system has so far successfully guided Ethiopia to design a project that has helped the country to systematically transition from less to more VAS integrated intervention. Government ownership & the clear framework for integration has contributed to the good results achieved so far. Recommendations are made to do a cost analysis to determine the direct costs of reaching children with VAS through integrated intervention.

Biography:
Professional experience in oncology circles, supported by multiple collaborations with major hospitals, allowed LOUIS CAZE to benefit from a reference fame as an Expert Support Care. First, Specialist lymphatic, muscular and circulatory LOUIS CAZE makes the total body approach his chosen field. It captures the benefits of physical-mental balance in the treatment of chronic diseases, and specializes in supportive care dedicated to cancer diseases. His collaboration with renowned organizations such as the Institute Gustave Roussy, was punctuated with exceptional professional and human experience. LOUIS CAZE is involved in implementing a comprehensive approach to cancer patient, in a real project of care that optimizes patient's quality of life, ensuring the different key aspects of health: the well physical and psychological, but also the entire social and family interactions. After these experiences, LOUIS CAZE work for the development of Support Care, including the management of pain and psychology.
Abstract:
What is supportive care?
Support care means all care and support that can be offered to a person suffering from cancer, alongside specific treatments to cure his disease such as chemotherapy, radiotherapy and surgery. They aim to Reduce the impact of disease and treatment. For this, a team of professionals specialized in very different fields put their skills available to patients to help them cope with this difficult time.The support can be offered care during and after treatment of the disease but also when the cancer treatments have no effect. They adapt to the needs of patients and their families.
What needs do they meet? The disease affects all aspects of daily life. The needs that may occur are numerous.
Support care can meet some of these needs:
• Primarily to control the symptoms related to the disease or its treatment
• In case of physical or psychological suffering
• To break isolation
• To learn to live with the physical consequences imposed sometimes disease
• To resume normal course of his life and benefit from the best possible living conditions, and that whatever the chances of recovery
• For many other reasons, each patient with the needs of its own.
Tamer M A Mohamed
Tenaya Therapeutics, USA
Title: Regulation of Cell Cycle to Stimulate Adult Cardiomyocyte Proliferation and Cardiac Regeneration

Biography:
Tamer Mohamed has completed his Ph.D. at the age of 30 years from University of Manchester, UK and held various Postdoctoral training at the University of Manchester, University of Glasgow, and University of Gottingen. He is currently conducting a collaborative research program between the University of Manchester and the J. David Gladstone Research Institute (UCSF) to identify novel therapies for heart failure. He has published more than 15 papers in reputed journals in the past 10 years and has been awarded several awards including the prestigious Young Investigator Award at the European Society of Cardiology Congress in 2010.
Abstract:
Background: Heart failure is often caused by loss of cardiac cells that are unable to re-enter the cell cycle for regeneration. Numerous attempts to identify such cell cycle regulators that could induce cell division of cardiomyocytes, or other cell types, have resulted in nuclear division (karyokinesis), but inefficient cleavage into two distinct daughter cells (cytokinesis) and subsequent survival. Such strategies stimulate cell cycle markers in no more than 1% of cardiomyocytes, limiting their utility.
Methods and results: Here, we took a combinatorial approach to screen for cell cycle factors and conditions that could recapitulate the fetal state of cardiomyocyte division. We found that ectopic introduction of the Cdk1/CyclinB1 and the Cdk4/CyclinD1 complexes promoted cell division in at least 15% of mouse and human cardiomyocytes in vitro. Rigorous assessment of cell division in vivo with the cardiac-specific (a-MHC) Cre-recombinase dependent Mosaic Analysis with Double Markers (MADM) lineage tracing system revealed similar efficiency in adult mouse hearts, leading to cardiac regeneration upon delivery of cell cycle regulators immediately after myocardial infarction and even one week after injury. This ability of cardiac regeneration resulted in significant improvement in cardiac function following acute or subacute myocardial infarction. Intra-myocardial injection of adenoviruses encoding the 4 cell cycle gene either injected at the time of the infarction or one week following myocardial infarction resulted in significant improvement in cardiac function as assessed by Echocardiography and MRI compared to animals received control virus. Furthermore, chemical inhibition of Tgfb and Wee1 made CDK1 and cyclin B dispensable, simplifying the minimal genetic requirement.
Conclusion: These findings reveal a discrete combination of genes that can unlock the proliferative potential in cells that had permanently exited the cell cycle.

Biography:
Ahmed Nasr Ghanem was qualified from Mansoura University, Egypt 1974. Passed is FRCS in 1983 and obtained his MD degree in 1988. He worked as Consultant Urologist Surgeon in Egypt, Saudi Arabia and United Kingdom. He reported over 40 publications. Now he is happily retired in Egypt.
Abstract:
The transurethral prostatectomy syndrome (TURS) is defined as severe vascular hypotension reaction that complicates endoscopic surgery as a result of massive irrigating fluid absorption causing severe acute dilution hyponatraemia (HN) of <120 mmol/l. The vascular shock is usually mistaken for one of the recognized shocks and Volumetric Overload Shock type 1 (VOS1) is overlooked making Volumetric Overload Shock Type 2 (VOS2) unrecognizable. In adults VOS1 is induced by the infusion of 3.5-5 litres of sodium-free fluids and is known as TURS or HN shock. VOS2 is induced by 12-14 litres of sodium-based fluids and is known as the adult respiratory distress syndrome. The most effective treatment for VOS1 and VOS2 is hypertonic sodium of 5%NaCl or 8.4% Sodium Bicarbonate. The literature on TURS is reviewed and the underlying patho-etiology is discussed. As Starling’s law for the capillary-interstitial fluid transfer, which underlies the principles of fluid therapy, proved wrong an alternative mechanism was found by studying the hydrodynamics of the porous orifice (G) tube akin to capillary. Incorporating the G tube in a chamber (C), representing the interstitial space surrounding a capillary, demonstrated a rapid dynamic magnetic field-like fluid circulation between the C and G tube lumen. The G-C phenomenon is autonomous having both filtration and absorption forces making a true replacement for Starling’s law in every tissue and organ of the body.
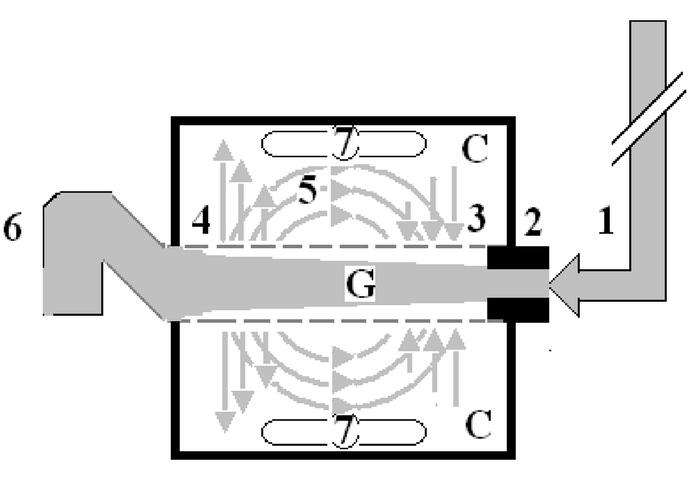
Figure: shows Diagram of the porous orifice (G) tube enclosed in chamber (C) based on several photographs demonstrating the magnetic field-like G-C circulation phenomenon. The proximal inflow (arterial) pressure (1) pushes fluid through the orifice (2) creating fluid jet in the lumen of the G tube. The fluid jet creates negative side pressure gradient causing suction maximal over the proximal half of the G tube near the inlet (3) that sucks fluid into lumen. The side pressure gradient turns positive pushing fluid out of lumen over the distal half maximally near the outlet (4). Thus the fluid around G tube inside C moves in magnetic field-like fluid circulation (5) taking an opposite direction to lumen flow of G. tube. The inflow (arterial) pressure (1) and orifice (2) induce the negative side pressure energy creating the dynamic G-C circulation phenomenon that is rapid, autonomous and efficient in moving fluid out from the G tube lumen at (4), irrigating C at (5), then sucking it back again at (3), maintaining net negative energy pressure (7) inside C. The distal outflow (venous) pressure (6) enhances outflow at (4) and its elevation may turn the negative energy pressure (7) inside C into positive, increasing volume and pressure inside C chamber.
Abdalla Taha
University of Modern Science, UAE
Title: Evaluation of mRNA markers for estimating blood deposition time

Biography:
Abdalla Taha has completed his Master degree at the age of 26 years from University of Modern Science and honor his Bachelor scholarship from Khalifa University in Abu Dhabi- UAE. I’m the Sales Manager of ECHO research & development S.r,l an Italian manufacturer. I had consult and design more than ten major research labs in different fields and working with UN for design 41 vocational schools. I awarded the second place in conference competition “Forensic Science: Preparing for Next Generation” in Amity University- Dubai- UAE. Currently I am in process of publishing my thesis master thesis about Evaluation of mRNA markers for estimating blood deposition time.
Abstract:
RNA analysis provide insight into diseases, molecular identification of body fluids and mechanisms leading to death and might develop into a valuable tool for identification of the cause of death in forensic pathology. Further potential uses are the determination of the age injuries of wounds and of the post-mortem interval. In this proof-of-concept pilot study clarifies and explains principles, applications and methods by offering a comprehensive and complete overview of using mRNA markers for estimating blood deposition time which can help to evaluate the time of the crime in forensic RNA work. Study presented in this thesis aimed to estimate the time passed since blood stains found in the crime scene by calculating the time of deposited blood using particular mRNA markers and unravelling one of the principles of at what time – when during the day or night a biological evidence was left at the scene – by applying the insights from circadian biology to some open forensic cases. By analyzing 4 candidate mRNA markers expression in peripheral blood samples collected from 29 health males. The four mRNA were collected from healthy persons for the duration of the 24 hours’ day/night interval under four different groups, i.e. night/early, morning early morning/morning, morning/afternoon and afternoon/night. This study identified 2 mRNAs with statistically significant expression rhythms which are MKNK2 and PER 3. It’s found that, in general mRNA-based estimation of time categories was less accurate. The value of mRNA was demonstrated for blood deposition timing and introduced a statistical model for estimating day/night time categories based on molecular biomarkers, which shall be further validated with additional samples in the future.








